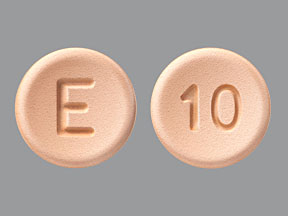
Opana ER 10 mg or the E 10 pill is an Extended-Release drug, which was approved in 2006 by the U.S. FDA to manage chronic pain. Endo Pharmaceuticals is a manufacturer of this medication. This medicine was not able to meet the standard of agency by considering it as abuse-deterrent. In 2011, the U.S. FDA approved the reformulation of Opana ER 10 mg tablet to make the drug resistant to abuse. The pharmaceutical company again reformulated in 2012. Endo Pharmaceuticals voluntarily removed their product from the market. Oxymorphone is an active ingredient as well as a generic variant that is found in this 10 mg pill. It is classified under Schedule II controlled substance (CII) which means it has a high potential for abuse. It should be prescribed by healthcare professionals. This medication comes in the form of oral tablets. You may take this drug orally without food (1 hour before food). Opana ER 10 mg pill is discontinued in the U.S. However, the Food and Drug Administration has approved its generic variants.
Function of Opana ER 10 mg Medication
Opana ER is a long-acting opioid pain reliever that works on the CNS (central nervous system) in the brain. This medicine has an affinity for the mu-opioid receptor which inhibits the pain pathway by reducing pain.
Side Effects of 10 mg Of Opana ER
After taking this medicine you may feel some of its side effects. The common side effects are dizziness, stomach pain, vomiting, etc. There are some adverse side effects which are hallucination, convulsions, difficulty in breathing, etc.
Precautions
- Do not crush or chew the drug.
- Alcohol should not be consumed while taking the yellow Opana ER pill.
- You should tell about your medical history to a healthcare provider.
- Due to drug interaction do not mix it with other drugs.












Reviews
There are no reviews yet.